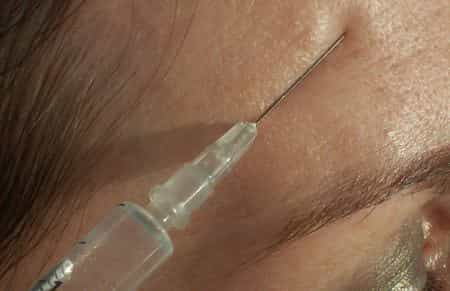
Botox is the best-known trade name of the pharmacological preparation that uses botulinum toxin, a neurotoxic protein produced by the bacterium Clostridium botulinum, as an active ingredient, for face lifting . Botulinum toxin is a double-chain polypeptide, with one 100 kDa chain disulfide-linked to another 50 kDa chain. The light chain is a protease enzyme that attacks one of the proteins (SNAP-25, syntaxin or synaptobrevin) of the neuromuscular junction, preventing the release of acetylcholine from the vesicles. By inhibiting the release of this neurotransmitter, the toxin interferes with nerve impulses and causes flaccid muscle paralysis characteristic of botulism and in contrast to the spastic paralysis seen in tetanus. In recent days, thanks to an article in the London newspaper The Sun, the other side of the coin has been brought to light, namely the dangers of Botox injections which in some cases can even lead to death. In England alone in the past year there would have been at least sixteen victims, including four minors. Doctors hypothesize that the cause of death is the fact that the botulinum toxin introduced into the body can expand into nearby tissues such as the esophagus, paralyzing or in any case severely limiting the organ's functions. The percentage of deceased patients resulting from the 55,000 injections is not high (less than one person in a thousand), but as requested by some of the consumer associations, it is necessary -rightly- to include all the adverse effects that can be expected in the contraindications to reach out to. In 1937 Alan B. Scott, an ophthalmologist at the Smith-Kettlewell Institute, used botulinum toxin A (BTX-A) in experiments on monkeys, and in 1980 he used BTX-A for the first time in humans for the treatment of strabismus. BTX-A was approved in 1989 by the Food and Drug Administration for the treatment of strabismus, blepharospasm, and hemifacial spasm in patients older than 12 years. In 2002, it received approval for use in aesthetic medicine for the temporary improvement of expression lines between the eyebrows (glabellar lines) through small skin injections that have a muscle-relaxing effect for up to 4 5 months. [more info ]
You may also like
Reduce cholesterol with fermented milk
Fermented milks with added phytosterols are able to reduce the level of bad cholesterol by about 10% in subjects with moderate hypercholesterolemia. This is demonstrated by the first meta-analysis carried out on about 400 people which analyzed the results of 3 studies already published in the literature on the effect of a fermented milk with… Continua a leggere Reduce cholesterol with fermented milk
Probiotic diet
With the arrival of the cold season, bacterial infections increase and therefore the use of antibiotics, which can alter the normal intestinal balance. Especially children and the elderly are at risk. It is therefore better to follow a " probiotic diet ", which includes the consumption of probiotics to be used not only during –… Continua a leggere Probiotic diet
20 years of Botox
It is a glorious story that of botox, discovered way back in 1820 and approved for the first time in 1989 by the American drug authority (FDA) with the indication for ocular dystonias. Since that year, the possible clinical applications have only multiplied up to the current over 20. But after its applications in ophthalmology,… Continua a leggere 20 years of Botox
Youth elixir
Another surprise to come regarding the enormous abilities of skin cells. Fibroblasts , which we had already mentioned because they are capable, united with an oocyte, of generating a stem cell, now ensure a long life for young and perfect skin. Goodbye to the old scalpel and annoying botulinum injections, it is now possible to… Continua a leggere Youth elixir
Botox for the nose
In an article last week, we talked about botulinum toxin for purely clinical use, in neuromuscular pathologies, for which treatment guidelines have just been defined. By now Botox is widely and easily used especially in cosmetic surgery and not only for wrinkles but also for touch-ups on the nose , through micro-injections with which the… Continua a leggere Botox for the nose