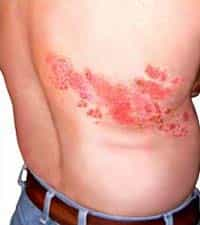
Herpes zoster, also known as "shingles", is the reactivation of the varicella zoster virus and consists of annoying and painful bubbles that cover the dermis. This pathology mainly affects the elderly (over 60 years of age) and more sporadically young people and adults. The treatment used up to now is the administration of Aciclovir, a known antiviral. However, from today the FDA has authorized the use of a new vaccine, called Zostavax , created specifically for people of a certain age. The effectiveness of the vaccine was tested on a sample of 38,000 people, divided equally between those who were injected with the drug and those who, as is now the practice in every test, were given a simple placebo. All this to evaluate the effective capacity of the medicine.

The results were surprising: in fact, a 50% decrease in cases was observed, rising up to 64% in patients aged between 60 and 69 years. Furthermore, in people affected by shingles, the duration of pain was halved. The side effects of Zostavax that have already been observed are: redness, pain and swelling around the injection site, along with itching and headaches.
You may also like
Business Phone Subscriptions: Guide to Costs, Options and Benefits
Choosing a business phone subscription can be a complex task, with numerous factors such as costs, benefits, and options to consider. This article explores various business phone subscriptions, examining the best deals and geographic cost variations to help businesses make informed decisions.
Private Mobile Phone Subscriptions: Finding the Best Fit for Your Needs
Selecting a mobile phone subscription can be daunting with myriad plans and hidden costs. This article explores various phone plans for private use, comparing prices and highlighting key considerations to help you choose the best mobile service provider.
Green Energy and Charging Stations: Proposals and Costs
As the world shifts towards greener energy sources, the demand for electric vehicle (EV) charging stations is on the rise. This article examines the current landscape of EV charging infrastructure, comparing proposals, costs, and benefits. We delve into geographic cost variations and spotlight the most competitive charging station offers.
Analysis of Green Energy Through Photovoltaic Panels
As the world searches for sustainable solutions to combat climate change, solar energy emerges as a frontrunner. This article explores the various proposals, costs, and advantages associated with photovoltaic panels, providing a comprehensive guide to understanding and investing in solar power. It also delves into geographical cost variations and compares current market offerings for optimal decision-making.